Abstract
Background: The combination of venetoclax (VEN) and a hypomethylating agent (HMA) has become the preferred first-line (1L) treatment for adults with acute myeloid leukemia (AML) who are unfit for intensive chemotherapy. Upon failure of VEN + HMA, outcomes are dismal, with a median overall survival (OS) of 2.4 months (Maiti et al., Haematologica, 2021). Molecularly targeted therapies represent a potential salvage strategy in patients (pts) harboring somatic alterations in FLT3, IDH1, or IDH2. This study describes outcomes for pts with FLT3- or IDH1/2-mutated AML who were treated with molecularly targeted therapy at the time of disease progression after VEN + HMA in the 1L setting.
Methods: This is a multi-center retrospective cohort analysis of pts with FLT3- or IDH1/2-mutated AML diagnosed between January 2018 and December 2021. FLT3 and IDH1/2 mutation status were determined via clinically validated genetic assays performed on bone marrow or peripheral blood samples. For all cases, demographic and clinical information were collected and analyzed. Responses were adjudicated as per European Leukemia Network 2017 response criteria. Survival analyses were performed using the Kaplan-Meier method; survival curves were compared using a log-rank test. Each collaborating center's institutional review board approved this study.
Results: Thirty individuals (9 female, 21 male) met inclusion criteria, with a median age of 74.5 years (inter-quartile range: 9.7 years) at the time of diagnosis. Nine pts carried mutations in FLT3 (7 internal tandem duplications [ITD], 2 tyrosine kinase domain mutations [TKD]) without co-occurring IDH1 or IDH2 mutations. Four pts carried IDH1 mutations alone and 11 carried IDH2 mutations alone. Five carried both a FLT3 mutation and an IDH2 mutation. One patient carried both an IDH1 and an IDH2 mutation.
Eighteen of 30 pts received frontline azacitidine + VEN, with the remaining 12 pts receiving decitabine + VEN, for an average of 6.1 cycles. The overall response rate was 86% in response-evaluable pts (24/28); 75% (21/28) had either a complete response (CR) or a CR with incomplete hematologic recovery (CRi). Twenty-two pts were treated with a targeted inhibitor of either FLT3 or IDH1/2 upon disease relapse (Table 1); the remaining 8 pts switched to targeted therapy due to either poor performance status or persistent cytopenias without evidence of relapse.
Six out of 9 pts with FLT3 mutations without concurrent IDH1 or IDH2 mutations received gilteritinib as salvage therapy following progression after VEN + HMA, while 3 received gilteritinib plus an HMA. Six pts with IDH2 mutations received IDH2-directed therapy upon relapse: 4/6 (67%) as monotherapy and 2/6 (33%) as enasidenib + HMA and enasidenib plus VEN + HMA, respectively. All 3 pts with IDH1 mutations were treated with IDH1 inhibitor monotherapy upon relapse. Overall, 15/22 (68%) pts received a targeted inhibitor as monotherapy and 7/22 (32%) received it in combination. Nineteen pts had response assessments following salvage therapy with targeted agents, with an ORR of 42% (8/19) and a median duration of response of 6.2 months (Table 1).
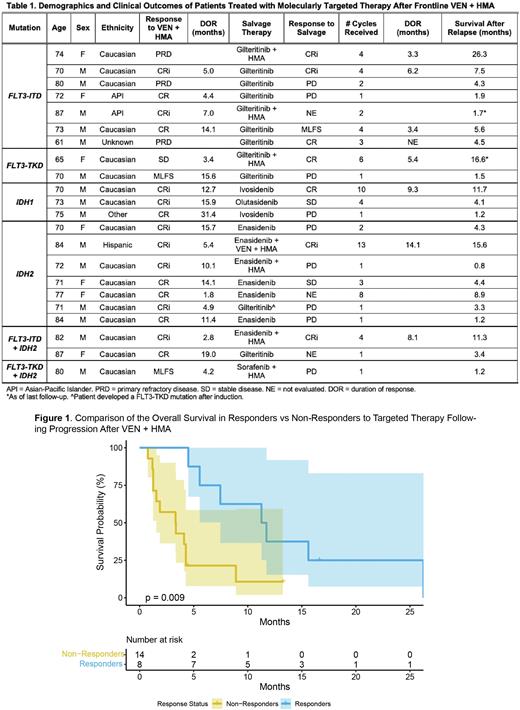
The median overall survival (mOS) following progression on 1L VEN + HMA was 4.4 months for all 22 pts. However, mOS was 11.5 months in responders to targeted therapy vs 3.3 months in non-responders (p = 0.009; Figure 1). In addition, mOS for pts receiving targeted therapy in combination was 15.6 months vs 4.3 months for those treated with targeted therapy alone (p = 0.017). For the 9 pts with FLT3 mutations, mOS following relapse was 5.6 months compared to 4.2 months for those with IDH1 or IDH2 mutations. In all, 8/22 (36%) pts went on to receive further salvage therapy following targeted therapy.
Conclusions: In this retrospective analysis, the use of targeted therapy as salvage treatment following frontline VEN + HMA was highly heterogeneous. In terms of mOS, targeted therapy compared favorably to historical data. Survival in responders to targeted therapy was superior to that of non-responders and pts treated with targeted therapy in combination with backbone therapies lived longer than those treated with targeted therapy alone. Further investigation is needed to determine the ideal sequencing and use of targeted therapies in this patient population.
Disclosures
Jonas:Genentech: Consultancy, Research Funding; AbbVie: Consultancy, Other: Travel Reimbursement, Research Funding; 47: Research Funding; BMS: Consultancy, Research Funding; GlycoMimetics: Consultancy, Other: protocol steering committee , Research Funding; Gilead: Consultancy, Other: data monitoring committee , Research Funding; Jazz: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Servier: Consultancy; Takeda: Consultancy; Tolero: Consultancy; Treadwell: Consultancy; Accelerated Medical Diagnostics: Research Funding; Amgen: Research Funding; AROG: Research Funding; BMS: Consultancy, Research Funding; Celgene: Research Funding; Daiichi Sankyo: Research Funding; F. Hoffmann-La Roche: Research Funding; Forma: Research Funding; Roche: Research Funding; Hanmi: Research Funding; Immune-Onc: Research Funding; Incyte: Research Funding; Loxo Oncology: Research Funding; LP Therapeutics: Research Funding; Pharmacyclics: Research Funding; Sigma Tau: Research Funding. Mannis:Abbvie: Consultancy; Agios: Consultancy; Astellas: Consultancy; BMS/Celgene: Consultancy; Genentech: Consultancy; Macrogenics: Consultancy; Pfizer: Consultancy; Stemline: Consultancy; Glycomimetics: Research Funding; Astex: Research Funding; Jazz: Research Funding; Forty Seven: Research Funding; Gilead: Research Funding; ImmuneOnc: Research Funding; Syndax: Research Funding; Servier: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal